In psychological assessment, different
measurement methods frequently yield inconsistent results. This is often evident
in research on male erectile function, where both self-report and physiological
measurements are used and where results from nocturnal penile tumescence (NPT)
monitoring can differ from results obtained using measures of waking-state
erectile performance ( Barry,
Blank, & Boileau, 1980 ; Karacan,
1970 ; Libman
& Fichten, 1987 ; Marshall,
Morales, Phillips, & Fenemore,1983 ).
Periodic nocturnal tumescence is
a naturally occurring phenomenon associated with the REM stage of the sleep
cycle in males of all ages. This fact has led researchers to investigate the
usefulness of NPT monitoring as a clinical tool in the evaluation of erectile
dysfunction ( Karacan,
1970 ). The procedure is based on the assumption that if erectile impairment
is psychogenic, the emotional, interpersonal, and attitudinal factors that
impair sexual responding during the waking state should generally be inoperative
during sleep, thereby allowing the normal pattern of nocturnal tumescence
to occur. In males with an organic basis for erectile dysfunction, a diminished
or absent sleep erection response would be expected.
There is some evidence that the
duration, frequency, and pattern of the NPT response can differentiate sexually
functional from dysfunctional males as well as an organic from a psychogenic
etiology for the erectile disorder (e.g., Marshall,
Surridge, & Delva, 1981 ). However, some estimate that up to 20% of
patients may be misclassified ( Schmidt
& Wise, 1981 ; Wasserman,Pollack,
Spielman, & Weitzman, 1980a ).
Recently, the assumption that nocturnal
and waking erections are neurophysiologically equivalent has come into question
(e.g., Bancroft
& Wu, 1983 ; Schiavi,
1988 ). Extreme differences in erectile capacity during waking and sleep
states in some organically impaired patients ( Nath
et al., 1981 ; Sakheim,
1985 ) and different topographical features in REM-related tumescence
and in daytime erectile responses ( Allen,
1981 ) have been found. Improvement in waking erectile capacity with no
concomittant NPT changes following treatment with an adrenergic blocking agent
( Condra,
Morales, Surridge, Owen, Marshall, & Fenemore, 1986 ) and differential
effects of androgen on NPT activity and erections evoked by visual stimuli
( Bancroft
& Wu, 1983 ) have also been demonstrated. These findings suggest that
the processes mediating erotically induced and REM related erections are not
the same.
It is common knowledge that both
quality of sleep and NPT may be disrupted by the testing procedure and that
inhibition of penile tumescence has been noted in normal men during REM periods
associated with dreams of high anxiety content ( Fisher,
1966 ; Karacan,
Goodenough, Shapiro, & Starker, 1966 ). Various psychiatric states,
such as depression, are related to diminished NPT responses, which are reversed
when the affective problem is resolved (e.g., Fisher
et al., 1979 ; Roose,
Glassman, Walsh, & Cullen, 1982 ; Thase
et al., 1987 ; Wasserman
et al., 1980b ). These data suggest that NPT cannot be assumed to bypass
psychological impediments to sexual responding.
Penile tumescence monitoring in
the waking state is a more recent procedure. Data indicate that men with psychogenic
erectile dysfunction achieved a significant degree of tumescence when assessed
with a procedure that included viewing an erotic film and self-stimulation
( Sakheim,
Barlow, & Beck, 1985 ). Sexually functional, organically impaired,
and psychogenically impaired men could be classified correctly with 80% accuracy
using this technique ( Sakheim,
1985 ). Because it is known that physiological and psychological factors
may affect both sleep and waking-state erections, it appears that waking-state
tumescence assessment constitutes a more direct and appropriate measure of
actual sexual performance. This conclusion is corroborated by the findings
of a recent study by Wincze and his associates, who examined erectile response
in relatively homogeneous diagnostic groups of dysfunctional men and in nondysfunctional
controls during both sleep and daytime assessment ( Wincze
et al., 1988 ). These investigators demonstrated that NPT results are
not representative of the maximum erectile response possible and that exposure
to erotic stimuli may override organic deficits in some cases.
Older men show significant age-related
declines in frequency,duration, and degree of sleep-related erectile episodes
( Kahn
& Fisher, 1969 ; Karacan,
Hursch, & Williams, 1972 ). Decrease in peak tumescence and a considerably
decreased rate of erectile response to erotic films in aging men have also
been documented ( Solnick
& Birren, 1977 ). Moreover, a marked increase in response threshold
to vibrotactile stimulation administered to the penis in aging (as well as
in diabetic) men relative to normal young men has been shown ( Rowland,
Greenleaf, Mas, & Davidson, 1987 ), and penile vibratory thresholds
were found to be related to sexual activity and erectile capacity. A range
of evaluation techniques has indicated increased impairment of erectile function
with increasing age (e.g., Libman
& Fichten,1987 ), although the etiology of the decline in sexual function
in aging men remains somewhat mysterious.
Despite interpretational difficulties,
NPT monitoring, which requires technical expertise and several nights in costly
sleep laboratory facilities, remains the most frequently used diagnostic test
of erectile disorder. A technique for assessment of erections that circumvents
many of the problems associated with the popular mercury strain gauge has
recently been developed by Dacomed. Marketed as the Snap-Gauge, it is a simple,
portable, and inexpensive behavioral measure of erectile functioning.
Although there are some Snap-Gauge
data for younger men, neither norms nor concordance with self-report measures
of sexual functioning are available for older individuals. Therefore, in the
present investigation we evaluated the relation between sleep Snap-Gauge results
and self-reports of waking-state erectile ability and sexual adjustment in
aging men.
Method
Subjects
Subjects were 58 aging married
men (mean age = 64 years, SD = 9, range = 50—79 )
and 19 of their wives. All participants were Caucasian, and there were approximately
equal numbers of Christian and Jewish subjects. Because this study forms part
of a larger investigation of the psychosexual consequences of transurethral
prostatectomy ( Libman,
Fichten, & Brender, 1987 ), all male subjects were suffering from
benign hypertrophy of the prostate and had been recommended for transurethral
prostatectomy by a urologist. Couples had been married for an average of 32
years SD = 12 ,
and the quality of marital relationships was generally satisfactory (mean
Locke-Wallace Marital Adjustment Scale score = 110, SD = 18
). Participants were generally in good physical and psychological
health (except for the prostatic symptoms). Subjects had an average of 13
years of education SD = 4 and
an average family income of $50,415 SD = $31,485 : this information,
combined with data on occupation, suggests that the socioeconomic status of
the sample was generally middle class.
Measures
In addition to the Snap-Gauge erectile
assessment device (and the evaluation of sleep quality) the measures included
self-report instruments in a range of areas. Socioeconomic, physical, and
psychological status were assessed to provide descriptive data for the sample.
Erectile capacity was operationalized as reported erectile performance in
a variety of individual and interpersonal sexual situations. Sexual expression
was more broadly examined in terms of individual and couple sexual satisfaction
and adjustment. These diverse aspects were examined to provide convergent
and concurrent validity data for the Snap-Gauge NPT measure. The specific
areas investigated and their associated measures were as follows:
1. Socioeconomic status and
personal and demographic variables.
A background information form designed
for this study asked for personal and demographic information such as age,
years of education, years married, whether retired or working, family income,
religion, and whether previous professional help had been sought for emotional,
sexual, or marital problems.
2. Physical status.
A physical symptoms checklist was
compiled for this study to evaluate general physical health. Information asked
for included the number of past and present illnesses, symptoms, and medications
used.
3. Psychological variables.
These were measured with the Brief
Symptom Inventory (BSI; Derogatis,
Rickels, & Rock, 1976 ), a self report psychological symptom inventory
designed for psychiatric and medical patients. Subjects indicate on a 5-point
scale the extent to which they are distressed by each of 53 psychological
and psychosomatic symptoms. It is a brief version of the Symptom Check List—90
(SCL—90), which is a frequently used instrument with good psychometric properties
( Derogatis,
1977 ).
4. Marital functioning.
The Kimmel
and Van der Veen (1974) version of the Locke-Wallace Marital Adjustment
Scale (MAS) was used to evaluate marital satisfaction. The MAS is a highly
reliable and well validated measure of marital adjustment ( Schiavi,
Derogatis, Kuriansky, O'Connor, & Sharpe, 1979 ). The Kimmel and Van
der Veen version contains 23 of the most significant items with scores weighted
to reflect current sex differences in patterns of responding.
5. Sexual functioning.
The following six measures of sexual
functioning were used:
Sexual History Form (SHF).
The SHF is a self-report sexual
history measure that utilizes a fixed alternative format and has been used
in sex therapy evaluation research by LoPiccolo and his colleagues. Typically
scored on an item-by-item basis, this measure has 28 variables. Some normative
data are available ( LoPiccolo,
Heiman, Hogan, & Roberts, 1985 ; Nowinski
& LoPiccolo, 1979 ).
Global Sexual Functioning Score.
To obtain a global evaluation of
male sexual functioning, scores on 12 SHF items (1, 2, 5, 12, 13, 14, 16,
17, 22, 23, 26, 27) that measure male sexual desire, sexual frequency, and
ability were proportioned, summed, and divided by 12 to provide a Global Sexual
Functioning score. Data indicate that the Global Sexual Functioning score
provides a good estimate of overall sexual functioning ( Creti,
Fichten, Libman, Takefman, & Brender, 1987 ). The maximum score is
I: thus lower scores indicate better sexual functioning.
Goals for Sex Therapy Scale
(GSTS).
This 15-item measure ( Lobitz
& Baker, 1979 ) uses a 7-point rating scale to evaluate satisfaction
with a man's ability to engage in various sexual activities. It yields one
score that reflects a man's satisfaction with his sex life. The instrument
has been shown to be sensitive to pre—post sex therapy changes ( Cohen,
Reynolds, Price, Schochet, & Anderson, 1980 ).
Sexual Self-Efficacy Scale–Erectile
Functioning ( SSES-E ).
The SSES-E measures
a man's belief that he could perform a variety of sexual behaviors. The scale
lists 25 desirable male sexual performance tasks; subjects designate those
they judge they could perform. For each designated item. subjects indicate
their level of confidence on a 10-point scale ranging from 10 ( quite uncertain
) to 100 ( certain ). The tasks include all 15 items from the Goals
for Sex Therapy Scale as well as additional items relevant to erectile functioning.
Strength of self-efficacy beliefs is provided by the mean confidence ratings
for all 25 tasks. The scale has demonstrated good reliability and validity
( Fichten,
Rothenberg, & Libman. 1988 ; Libman,
Rothenberg, Fichten, & Amsel, 1985 ).
Sexual Interaction Inventory
(SII).
This instrument, compiled by LoPiccolo
and Steger (1974) , is the most frequently used measure of sexual satisfaction.
It consists of a list of 17 heterosexual couple behaviors. For each behavior,
subjects answer six questions using a 6-point scale. The scores of each partner
are used to derive a profile on five subscales for each partner as well as
a couple summary score (Scale 6). In the present investigation only Scale
6 (Total Disagreement) was used; it provides an overall summary score for
the couple and measures total disharmony and dissatisfaction in the sexual
relationship. The lower the score, the greater the sexual harmony. The test
is reliable on test-retest and has good internal consistency; also, all scales
have been shown to be correlated with self-report of sexual satisfaction.
The measure has been demonstrated to be reactive to treatment and able to
discriminate sexually dysfunctional clients from nonclients ( LoPiccolo
& Steger, 1974 ).
Dacomed Snap-Gauge Impotence
Testing Device (Snap-Gauge).
The Snap-Gauge consists of a small
velcro band that incorporates three preset plastic snaps with different release-force
constants. Penile tumescence and rigidity are determined by whether the subject
breaks none, one, two, or all three of the snaps ( Ek,
Bradley, & Krane, 1983 ). It measures not only change in penile circumference
but also, indirectly, the rigidity of erections. Data indicate that Snap-Gauge
results correlate highly with technician-assessed quality of nocturnal tumescence
( Ellis,
Doghramji, & Bagley, 1988 ). Satisfactory reliability and validity
have been reported for the Snap-Gauge for nonelderly samples ( Anders,
Bradley, & Krane, 1983 ; Carter,
1983 ).
6. Sleep quality.
A 3-item sleep questionnaire was
developed for the present study. It asked subjects to rate (a) how satisfactorily
they had slept ( well or poorly ), (b) approximately how many
hours they had slept, and (c) approximately how many hours they normally sleep.
Procedure
Subjects were recruited through
urologists practicing at six Montreal hospitals. A test battery that included
the self-report measures listed above was administered to subjects in two
sessions as soon as possible after the date for the prostatectomy was set.
Subjects were instructed on the Snap-Gauge technique (and given a copy of
the instructions to take home) after the first testing session. They were
requested to use the device for one night, to complete the sleep questionnaire
for the night it was used, and to return both at the second testing appointment.
We discovered during the pilot
phase of our study that the terminology used in the Dacomed instructions was
somewhat anxiety provoking for our aging male subjects. For example, the term
"Snap-Gauge" often elicited an expression of alarm and the question, "Snap
what?". The phrase "impotence testing" was used frequently in the Dacomed
instructions, and the probability of a physical cause for erectile problems
was made fairly salient. Therefore, we modified the terminology some what.
We called the device an "Expansion Tape" and explained that "sometimes males
experience some degree of erection from time to time during the night while
they are sleeping.... This tape gives us some indication of whether this is
happening.... We are evaluating whether the tape is useful."
Results
Age and Snap-Gauge Changes
Twenty-nine of the 58 subjects
(50%) broke no snaps. Of the remaining subjects, 11 (19%) broke one snap,
9 (16%) broke two snaps, and 9 (16%) broke all three. When subjects were divided
into younger (age less than 65) and older (age equal to or greater than 65)
groups (mean age of younger subjects = 58; older = 70), results indicated
that younger subjects broke more snaps M
= 1.45 than did older subjects M
= 0.41 , t 56
= 3.89, p < .001 .
t-Test Comparisons
To assess differences between subjects
who broke no snaps and those who broke at least one snap, a discriminant analysis
on the two groups was carried out. This was done in order to screen for the
t tests used to evaluate differences in diverse aspects of sexual adjustment
(the SII global summary was excluded because including it would have meant
a substantial drop in sample size because it requires a couple score). This
showed a significant difference, x 2 16,
N = 51 = 27.60, p < .05 (based on Wilks's
lambda). The series of t tests (means are detailed in Table
1 ) shows that there were no significant differences on nonsexual variables
and few significant findings on sexual variables. Before making a Bonferroni
adjustment to the alpha levels, we found significant differences on only 3
of the 13 sexual variables: frequency of morning erections, t
53 = 2.25, p < .05 ;
frequency of sexual arousal, t 53
= 3.39, p < .001 ; and variety in couple sexual
repertoire, t 52 = 2.47,
p < .05 . In addition, we found marginally significant
differences on sexual self-efficacy, t 53 = 1.96, p < .10 , and on frequency of
inter-course and couple sexual activity, t 53 = 1.70, p < .10 . After a Bonferroni
adjustment, the only significant difference that remained was on the frequency
of sexual arousal.
Because such results may have been
due to grouping subjects with marginal nocturnal erections (i.e., those who
broke only one snap) with those who had better erections (i.e., those who
broke two or three snaps), we performed another series of t tests to
compare the scores of subjects who broke no snaps and those who broke at least
two; the discriminant analysis here was marginally significant, x
2 16, N = 40 = 24.08, p < . 10 . As
the means in Table
1 and the t -test results show, significant differences before
the Bonferroni adjustment were found only on the following variables: frequency
of morning erections, t 42 =
2.70, p < .01 ; frequency of sexual arousal, t
42 = 2.99, p < .01 ;
and sexual self-efficacy, t 42
= 2.04, p < .05 . The comparison on variety in
couple sexual repertoire was marginally significant, t 41 = 1.85, p < .10 . As in the
previous series of t tests, after the Bonferroni adjustment, only the
test on frequency of sexual arousal remained significant.
Chi-Square Comparisons
Because analyses detailed in Table
1 were carried out on mean scores, it was not possible to determine from
the data what percentage of the subjects who did break snaps (and what percentage
of those who did not break any snaps) were functioning well on the variables
of interest. Therefore, a series of chi-square tests was performed to assess
the association between breaking snaps and quality of sexual functioning and
adjustment. Mean scores were used to split the sample into those who functioned
better and worse than average on the variables of interest. Again, we made
separate comparisons on subjects who broke at least one snap and on subjects
who broke at least two.
Frequencies in Table
2 and the chi-square tests show consistent, significant p < .05 findings only on the following
variables: age, frequency of morning erections, and frequency of sexual arousal.
After the Bonferroni adjustment to the alpha level, the chi-square test was
significant only for age.
Sleep Quality and Quantity
Because sleep quality and quantity
may have affected the number of snaps broken, a series of analyses on sleep
parameters was carried out. On the night of Snap-Gauge testing, subjects slept
an average of 6.7 hours SD = 1.6 ; the usual number of hours
of sleep was 7.1 SD = 0.9 .
Only 3 of the 36 subjects who provided sleep data slept at least 1 hour less
than normal on the Snap-Gauge night. Neither t -test nor chi-square-test
results showed a significant relationship between quantity of sleep and number
of snaps broken.
To ascertain whether quality of
sleep influenced the results, we compared the Snap-Gauge scores of the 6 subjects
who "slept poorly" M = 1.0, SD = 1.3 with those of the
29 who "slept well" M = 1.0, SD
= 1.2 ; again, neither t tests nor chi- square
tests revealed any significant differences.
Discussion
The results indicate that (a) only
50% of the sample broke any snaps during Snap-Gauge NPT assessment,
(b) younger men broke more snaps than older men, (c) men who broke no snaps
had consistently worse scores on the self-reported frequencies of sexual arousal
and of morning erections than men who did break snaps. These two groups (d)
did not differ on physical health or on psychological or marital adjustment.
It is noteworthy that they did not differ consistently and significantly
on most of the sexual variables investigated.
In the present study, NPT was measured
by the Snap-Gauge technique administered on only 1 testing night. This represents
a departure from the recommended procedure of testing on 2 or 3 nights. One
cannot be sure that an optimal measure of sleep erection capacity has been
obtained by sampling only 1 night. In addition, it is not possible to obtain
detailed information about the number of erections or about their duration
using the Snap-Gauge. Nevertheless, it is interesting that our results parallel
the age-related impairment of sleep erections measured by polygraphic recording
in sleep laboratories (i.e., older men in our sample demonstrated an impaired
Snap-Gauge "score" relative to younger men). '"Younger" aging men report a
higher frequency of morning erections and greater sexual desire than do "older"
aging men ( Libman,
1989 ; Libman
et al., in press ); this provides additional validity for the Snap-Gauge
as a measure of sleep erectile capacity. Furthermore, performance on
the Snap-Gauge was related to reported sexual desire, indicating that Snap-Gauge
NPT data are related to at least some aspect of subjective sexual experience
in the waking state in our sample of aging men. In view of these results,
the present negative findings (which suggest a general lack of concordance
between Snap-Gauge NPT results and self-report measures of waking-state erectile
ability, sexual performance, and adjustment) assume increased importance.
Data from the present investigation
suggest that NPT, as measured by the Snap-Gauge, does not constitute a valid
measure of daytime erectile ability in the present sample. For example,
it is important to note that when waking-state sexual expression was examined
more broadly, men who differed in their Snap-Gauge NPT performance showed
no consistent differences in (a) global sexual functioning, (b) reported frequency
of actual or desired couple sexual activity, (c) variety in couple sexual
repertoire, or (d) subjective satisfaction with their own sexual capacities
or with the couple sexual relationship. Perhaps most important, they did not
differ significantly on (e) self-reported difficulty obtaining or maintaining
erections during sexual activities.
The present findings cast further
doubt on the assumption that sleep and waking-state erections are "equivalent"
and suggest that the use of NPT to evaluate the multidimensional aspects of
erectile ability and sexual adjustment in aging men may be questionable. As
a measure of general capacity for sexual expression in the older men of our
sample, Snap-Gauge NPT certainly did not provide a sufficiently accurate reflection
of waking-state adjustment. As the findings of Wincze
et al. (1988) demonstrated in a younger sample, it seems that determination
of organic versus psychogenic etiology for erectile dysfunction, especially
in older men, cannot be based on even a detailed examination of NPT parameters
alone. The etiology of "normal" age-related changes in the pattern of sleep-related
erections has not yet been identified, and the results of the present study
indicate minimal concordance between "impairment" assessed using Snap-Gauge
NPT and waking-state sexual adjustment.
Typically, NPT data demonstrate
a recognizable and consistent pattern for sexually well functioning men and
a more variable pattern for men reporting erectile difficulties (see Anders
et al., 1983 , for an example using the Snap-Gauge technique). Minimal
data exist on concordance between NPT performance and more broadly defined
measures of sexual expression for males in any age category.
In order to expand the generalizability
of findings from the present study, future research should sample a representative
group of "healthy" aging men. It is difficult to locate such a sample in an
aging population, because surgeries, illnesses, and frequent medication are
common. Moreover, a totally healthy group may constitute an atypical sample
of aging individuals. A more appropriate sampling technique might be selecting
multiple homogeneous samples, similar to the one used in the present study,
but with another commonly experienced and age-related surgery (e.g., hernia
repair, cataract surgery).
The Snap-Gauge is suitable for
nocturnal or daytime use and can be used in familiar home surroundings as
well as in the laboratory. These qualities permit maximum convenience in verifying
and amplifying the present preliminary findings. Testing should be carried
out on at least 2 consecutive nights for greater reliability. In addition,
the technique should be used in the context of exposure to erotic stimuli
in the waking state. Finally, data from physiological measures of erectile
ability obtained under sleep and waking-state conditions should be examined
in relation to more broadly defined aspects of waking-state sexual capacity,
expression, and performance. This would allow for comparisons of sleeping
and waking-state erectile response and would relate Snap-Gauge performance
to other measures of sexual adjustment and expression.
References
Allen, R. (1981). Erectile impotence: Objective diagnosis from
sleep related erections (nocturnal penile tumescence). Journal of Urology,
126, 353
Allen, R. (1981). Erectile impotence: Objective diagnosis from
sleep related erections (nocturnal penile tumescence). Journal of Urology,
126, 353
Anders, E. K., Bradley, W. E. & Krane, R. J. (1983). Nocturnal
penile rigidity measured by the Snap-Gauge Band. Journal of Urology, 129,
964-966.
Anders, E. K., Bradley, W. E. & Krane, R. J. (1983). Nocturnal
penile rigidity measured by the Snap-Gauge Band. Journal of Urology, 129,
964-966.
Bancroft, J. & Wu, F. C. W. (1983). Changes in erectile
responsiveness during androgen replacement therapy. Archives of Sexual
Behavior, 12, 59-66.
Bancroft, J. & Wu, F. C. W. (1983). Changes in erectile
responsiveness during androgen replacement therapy. Archives of Sexual
Behavior, 12, 59-66.
Barry, J. M., Blank, B. & Boileau, M. (1980). Nocturnal
penile tumescence monitoring with stamps. Urology, 15, 171-172.
Barry, J. M., Blank, B. & Boileau, M. (1980). Nocturnal
penile tumescence monitoring with stamps. Urology, 15, 171-172.
Carter, K. H. (1983). Clinical experience summary of the
Dacomed Snap-Gauge for quantitative measurement of penile rigidity .(Minneapolis,
MN: Dacomed Corporation)
Cohen, B. D., Reynolds, B. S., Price, S., Schochet, B. V. & Anderson,
A. J. (1980, September). Group treatment for erectile dysfunction in non-partnered
males: New findings .(Paper presented at the 88th annual convention of
the American Psychological Association, Montreal, Quebec, Canada)
Condra, M., Morales, A., Surridge, D. H., Owen, J. A., Marshall,
P. & Fenemore, J. (1986). The unreliability of nocturnal penile tumescence
recording as an outcome measurement in the treatment of organic impotence.
Journal of Urology, 135, 280-282.
Condra, M., Morales, A., Surridge, D. H., Owen, J. A., Marshall,
P. & Fenemore, J. (1986). The unreliability of nocturnal penile tumescence
recording as an outcome measurement in the treatment of organic impotence.
Journal of Urology, 135, 280-282.
Creti, L., Fichten, C. S., Libman, E., Takefman, J. & Brender,
W. (1987, November). A global score for the "Sexual History Form" and its
effectiveness .(Paper presented at the annual convention of the Association
for Advancement of Behavior Therapy, Boston, MA)
Derogatis, L. R. (1977). The Psychopathology Rating Scale: A brief
description .(Unpublished manuscript, Johns Hopkins University School
of Medicine, Baltimore)
Derogatis, L. R., Rickels, K. & Rock, A. F. (1976). The
SCL-90 and the MMPI: A step in the validation of a new self-report scale.
British Journal of Psychiatry, 128, 280-289.
Derogatis, L. R., Rickels, K. & Rock, A. F. (1976). The SCL-90
and the MMPI: A step in the validation of a new self-report scale. British
Journal of Psychiatry, 128, 280-289.
Ek, A., Bradley, W. F. & Krane, R. J. (1983). Snap-Gauge
Band: New concept in measuring penile rigidity. Urology, 21, 63-67.
Ek, A., Bradley, W. F. & Krane, R. J. (1983). Snap-Gauge
Band: New concept in measuring penile rigidity. Urology, 21, 63-67.
Ellis, D. J., Doghramji, K. & Bagley, D. H. (1988). Snap-Gauge
Band versus penile rigidity in impotence assessment. The Journal of Urology,
140, 61-63.
Ellis, D. J., Doghramji, K. & Bagley, D. H. (1988). Snap-Gauge
Band versus penile rigidity in impotence assessment. The Journal of Urology,
140, 61-63.
Fichten, C. S., Rothenberg, I. & Libman, E. (1988). Sexual
Self-Efficacy Scale–Erectile Functioning.(In W. L. Yarber & C. M. Davis
(Eds.), Sexuality related measures: A compendium (pp. 129—131). Lake
Mills, IA: Graphic Publishing.)
Fisher, C. (1966). Dreaming and sexuality.(In R. M. Loewenstein,
L. M. Newman, & M. Schur (Eds.), Psychoanalysis: A general psychology:
Essays in the honor of Heinz Hartman (pp. 537—563). New York: International
Universities Press.)
Fisher, C., Schiavi, R. C., Edwards, A., Davis, D. M., Reitman, M.
& Fine, J. (1979). Evaluation of nocturnal penile tumescence in the differential
diagnosis of sexual impotence: A quantitative study. Archives of General
Psychiatry, 36, 431-437.
Fisher, C., Schiavi, R. C., Edwards, A., Davis, D. M., Reitman,
M. & Fine, J. (1979). Evaluation of nocturnal penile tumescence in the
differential diagnosis of sexual impotence: A quantitative study. Archives
of General Psychiatry, 36, 431-437.
Kahn, E. & Fisher, C. (1969). The sleep characteristics of the
normal aged male. Journal of Nervous and Mental Disease, 148, 477-494.
Kahn, E. & Fisher, C. (1969). The sleep characteristics
of the normal aged male. Journal of Nervous and Mental Disease, 148,
477-494.
Karacan, I. (1970). Clinical value of nocturnal erections
in the prognosis and diagnosis of impotence. Medical Aspects of Human Sexuality,
4, 27-34.
Karacan, I. (1970). Clinical value of nocturnal erections
in the prognosis and diagnosis of impotence. Medical Aspects of Human Sexuality,
4, 27-34.
Karacan, I., Goodenough, D. R., Shapiro, A. & Starker,
S. (1966). Erection cycle during sleep in relation to dream anxiety. Archives
of General Psychiatry, 15, 183-189.
Karacan, I., Goodenough, D. R., Shapiro, A. & Starker,
S. (1966). Erection cycle during sleep in relation to dream anxiety. Archives
of General Psychiatry, 15, 183-189.
Karacan, I., Hursch, C. J. & Williams, R. L. (1972). Some characteristics
of nocturnal penile tumescence in elderly males. Journal of Gerontology,
27, 39-45.
Karacan, I., Hursch, C. J. & Williams, R. L. (1972). Some
characteristics of nocturnal penile tumescence in elderly males. Journal
of Gerontology, 27, 39-45.
Kimmel, D. & Van der Veen, F. (1974). Factors of marital
adjustment in Locke's Marital Adjustment Test. Journal of Marriage and
the Family, 29, 57-63.
Kimmel, D. & Van der Veen, F. (1974). Factors of marital
adjustment in Locke's Marital Adjustment Test. Journal of Marriage and
the Family, 29, 57-63.
Libman, E. (1989). Sociocultural and cognitive factors in
aging sexual expression: Conceptual and research issues. Canadian Psychology,
30, 560-567.
Libman, E. (1989). Sociocultural and cognitive factors in
aging sexual expression: Conceptual and research issues. Canadian Psychology,
30, 560-567.
Libman, E. & Fichten, C. S. (1987). Prostatectomy and sexual
function: A review. Urology, 29, 467-478.
Libman, E. & Fichten, C. S. (1987). Prostatectomy and
sexual function: A review. Urology, 29, 467-478.
Libman, E., Fichten, C. S. & Brender, W. (1987). Prostatectomy
and sexual function in the aging male .(Final report to the Conseil Québécois
de la Recherche Sociale (ISBN 2-550-17809-2))
Libman, E., Fichten, C. S., Creti, L., Weinstein, N., Amsel,
R. & Brender, W. (in press). Transurethral prostatectomy: Differential
effects of age category and presurgery sexual functioning on postprostatectomy
sexual adjustment. Journal of Behavioral Medicine., ,
Libman, E., Fichten, C. S., Creti, L., Weinstein, N., Amsel,
R. & Brender, W. (in press). Transurethral prostatectomy: Differential
effects of age category and presurgery sexual functioning on postprostatectomy
sexual adjustment. Journal of Behavioral Medicine., ,
Libman, E., Rothenberg, I., Fichten, C. S. & Amsel, R.
(1985). SSESE: A measure of sexual self-efficacy in erectile functioning.
Journal of Sex and Marital Therapy, 11, 233-244.
Libman, E., Rothenberg, I., Fichten, C. S. & Amsel, R. (1985).
SSESE: A measure of sexual self-efficacy in erectile functioning. Journal
of Sex and Marital Therapy, 11, 233-244.
Lobitz, W. C. & Baker, E. L., (1979). Group treatment
of single males with erectile dysfunction. Archives of Sexual Behavior,
8, 127-138.
Lobitz, W. C. & Baker, E. L., (1979). Group treatment
of single males with erectile dysfunction. Archives of Sexual Behavior,
8, 127-138.
LoPiccolo, J., Heiman, J., Hogan, D. R. & Roberts, C. W. (1985).
Effectiveness of single therapists versus cotherapy teams in sex therapy.
Journal of Consulting and Clinical Psychology, 53, 287-294.
LoPiccolo, J., Heiman, J., Hogan, D. R. & Roberts, C. W. (1985).
Effectiveness of single therapists versus cotherapy teams in sex therapy.
Journal of Consulting and Clinical Psychology, 53, 287-294.
LoPiccolo, J. & Steger, J. (1974). The Sexual Interaction Inventory:
A new instrument for assessment of sexual dysfunction. Archives of Sexual
Behavior, 3, 585-595.
LoPiccolo, J. & Steger, J. (1974). The Sexual Interaction
Inventory: A new instrument for assessment of sexual dysfunction. Archives
of Sexual Behavior, 3, 585-595.
Marshall, P., Morales, A., Phillips, P. & Fenemore, J. (1983).
Nocturnal penile tumescence with stamps: A comparative study under sleep laboratory
conditions. Journal of Urology, 130, 88-89.
Marshall, P., Morales, A., Phillips, P. & Fenemore, J. (1983).
Nocturnal penile tumescence with stamps: A comparative study under sleep laboratory
conditions. Journal of Urology, 130, 88-89.
Marshall, P., Surridge, D. & Delva, N. (1981). The role of nocturnal
penile tumescence in differentiating between organic and psychogenic impotence:
The first stage of validation. Archives of Sexual Behavior, 10, 1-10.
Marshall, P., Surridge, D. & Delva, N. (1981). The role
of nocturnal penile tumescence in differentiating between organic and psychogenic
impotence: The first stage of validation. Archives of Sexual Behavior,
10, 1-10.
Nath, R., Menzoian, J., Kaplan, K., Millian, M., Siroky, T.
& Krane, R. (1981). The multidisciplinary approach to vasculogenic impotence.
Surgery, 89, 124-133.
Nath, R., Menzoian, J., Kaplan, K., Millian, M., Siroky, T.
& Krane, R. (1981). The multidisciplinary approach to vasculogenic impotence.
Surgery, 89, 124-133.
Nowinski, J. K. & LoPiccolo, J. (1979). Assessing sexual
behavior in couples. Journal of Sex and Marital Therapy, 5, 225-243.
Nowinski, J. K. & LoPiccolo, J. (1979). Assessing sexual
behavior in couples. Journal of Sex and Marital Therapy, 5, 225-243.
Roose, S. P., Glassman, A. H., Walsh, T. & Cullen, K.
(1982). Reversible loss of nocturnal penile tumescence during depression:
A preliminary report. Neuropsychobiology, 8, 284-288.
Roose, S. P., Glassman, A. H., Walsh, T. & Cullen, K. (1982).
Reversible loss of nocturnal penile tumescence during depression: A preliminary
report. Neuropsychobiology, 8, 284-288.
Rowland, D. L., Greenleaf, W. J., Mas, M. & Davidson,
J. M. (1987). Penile and finger sensory thresholds in aging and diabetes
.(Unpublished manuscript)
Sakheim, D. K. (1985). Waking assessment of erectile potential:
The validation of a laboratory procedure to aid in the differential diagnosis
of psychogenic and organic impotence .(Unpublished doctoral dissertation,
State University of New York at Albany)
Sakheim, D. K., Barlow, D. H. & Beck, J. G. (1985). Diurnal
penile tumescence: A pilot study of waking erectile potential in sexual functional
and dysfunctional men. Sexuality and Disability, 4, 68-97.
Sakheim, D. K., Barlow, D. H. & Beck, J. G. (1985). Diurnal
penile tumescence: A pilot study of waking erectile potential in sexual functional
and dysfunctional men. Sexuality and Disability, 4, 68-97.
Schiavi, R. C. (1988). Nocturnal penile tumescence in the
evaluation of erectile disorders: A critical review. Journal of Sex and
Marital Therapy, 14, 83-97.
Schiavi, R. C. (1988). Nocturnal penile tumescence in the
evaluation of erectile disorders: A critical review. Journal of Sex and
Marital Therapy, 14, 83-97.
Schiavi, R. C., Derogatis, L. R., Kuriansky, J., O'Connor,
D. & Sharpe, L. (1979). The assessment of sexual function and marital
interaction. Journal of Sex and Marital Therapy, 5, 169-224.
Schiavi, R. C., Derogatis, L. R., Kuriansky, J., O'Connor,
D. & Sharpe, L. (1979). The assessment of sexual function and marital
interaction. Journal of Sex and Marital Therapy, 5, 169-224.
Schmidt, H. & Wise, H. (1981). Significance of impaired penile
tumescence and associated polysomnographic abnormalities in the impotent patient.
Journal of Urology, 126, 348-352.
Schmidt, H. & Wise, H. (1981). Significance of impaired
penile tumescence and associated polysomnographic abnormalities in the impotent
patient. Journal of Urology, 126, 348-352.
Solnick, R. L. & Birren, J. E. (1977). Age and male erectile
responsiveness. Archives of Sexual Behavior, 6, 1-9.
Solnick, R. L. & Birren, J. E. (1977). Age and male erectile
responsiveness. Archives of Sexual Behavior, 6, 1-9.
Thase, M. E., Reynolds, C. F., Glanz, L. M., Jennings, J. R., Sewitch,
D. E., Kupfer, D. J. & Frank, E. (1987). Nocturnal penile tumescence in
depressed men. American Journal of Psychiatry, 144, 89-92.
Thase, M. E., Reynolds, C. F., Glanz, L. M., Jennings, J. R., Sewitch,
D. E., Kupfer, D. J. & Frank, E. (1987). Nocturnal penile tumescence in
depressed men. American Journal of Psychiatry, 144, 89-92.
Wasserman, M. D., Pollak, C. P., Spielman, A. J. & Weitzman,
E. D. (1980a). The differential diagnosis of impotence. The measurement of
nocturnal penile tumescence. Journal of the American Medical As sociation,
243, 2038-2042.
Wasserman, M. D., Pollak, C. P., Spielman, A. J. & Weitzman,
E. D. (1980a). The differential diagnosis of impotence. The measurement of
nocturnal penile tumescence. Journal of the American Medical As sociation,
243, 2038-2042.
Wasserman, M. D., Pollak, C. P., Spielman, A. J. & Weitzman,
E. D. (1980b). Theoretical and technical problems in the measurement of nocturnal
penile tumescence for the differential diagnosis of impotence. Psychosomatic
Medicine, 42, 575-585.
Wasserman, M. D., Pollak, C. P., Spielman, A. J. & Weitzman,
E. D. (1980b). Theoretical and technical problems in the measurement of nocturnal
penile tumescence for the differential diagnosis of impotence. Psychosomatic
Medicine, 42, 575-585.
Wincze, J. P., Bansal, S., Malhotra, C., Balko, A., Susset,
J. G. & Malamud, M. (1988). A comparison of nocturnal penile tumescence
and penile response to erotic stimulation during waking states in comprehensively
diagnosed groups of males experiencing erectile difficulties. Archives
of Sexual Behavior, 17, 333-348.
This study was funded by a grant from the Conseil Québécois de la Recherche
Sociale.
We are grateful to the following urologists for their support: S. Jacobson,
M. M. Elhilali, S. Aronson, Y. Taguchi, and M. Macramella.
Correspondence may be addressed to Eva Libman, Department of Psychiatry,
Sir Mortimer B. Davis–Jewish General Hospital, 3755 Côte Ste-Catherine Road,
Montréal, Québec, Canada, H3T 1E2.
Received: November 29, 1988
Revised: May 3, 1989
Accepted: May 17, 1989
Table 1. Snap-Gauge
Results and Mean Scores on other Variables
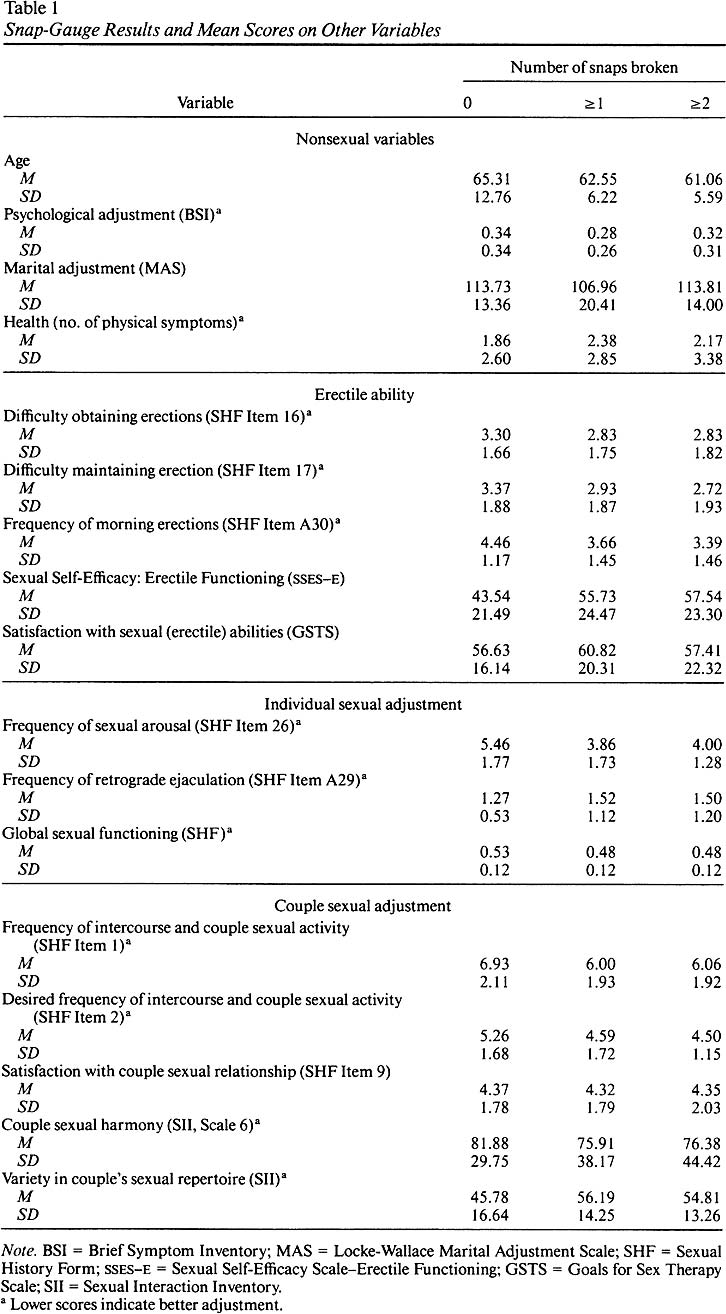
Table 2. Snap-Gauge Performance and Number of Subject Functioning
Well or Poorly on Other Variables


